Dr. Todd Hudnall
Professor
|
Office: CHEM 313 Phone: (512) 245-4443 Fax: (512) 245-2374 Email: th40@txstate.edu |
Educational Background
|
Honors and Awards
|
|
Areas of InterestMain Group Organometallic Chemistry |
|
|
Related Web Sites |
Research in the Hudnall Group
Recent Publications
Ledet, A. D. and Hudnall, T. W.* Reduction of a Diamidocarbene-Supported Borenium Cation: Isolation of a Neutral Boryl-Substituted Radical and a Carbene-Stabilized Aminoborylene Dalton Trans. 2016, Accepted Article (invited manuscript for the 2016 themed issue – New Talent: Americas). Link to Article
Hudnall, T. W.*; Dorsey, C. L.; Jones, J. S.; Gabbaï, F. P.* Stepwise Reduction of an α-Phosphonio-Carbocation to a Crystalline Phosphorus Radical Cation and an Acridinyl-Phosphorus Ylide Chem – Eur. J. 2016, Accepted Article. Link to Article Featured on Frontispiece
Deardorff, C. L.; Sikma, R. E.; Rhodes, C. P.; Hudnall, T. W.* Carbene-derived α-acyl formamidinium cations: organic molecules with readily tunable multiple redox processes Chem. Commun. 2015, Accepted Article (invited manuscript for the 2016 Emerging Investigators special issue). Link to Article
Dorsey, C. L.; Mushinski, R. M.; Hudnall, T. W.* Metal-Free Stabilization of Monomeric Antimony(I): A Carbene-Supported Stibinidene. Chem. – Eur. J. 2014, 20, 8914-8917. Link to Article
Rodrigues, R. R.; Dorsey, C. L.; Arceneaux, C. A.; Hudnall T. W.* Phosphaalkene vs. Phosphinidene: The Nature of the P–C Bond in Carbonyl-Decorated Carbene→PPh Adducts. Chem. Commun. 2014, 50, 162-164. Link to article Featured on back cover
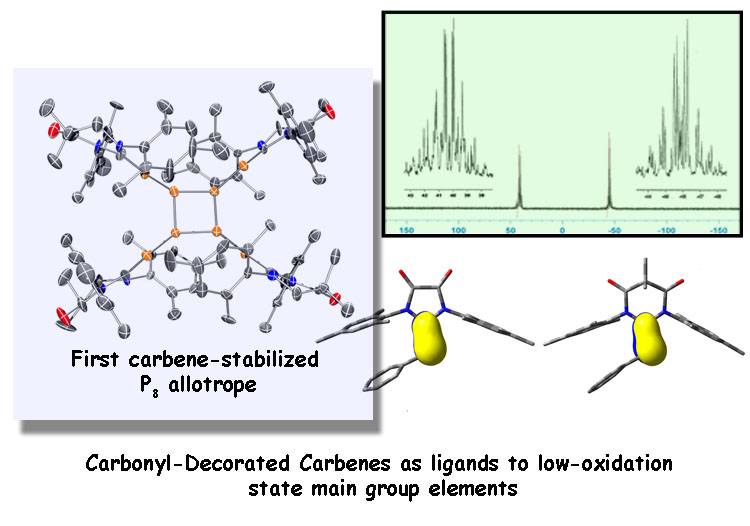
Dorsey, C. L.; Squires, B. M.; Hudnall, T. W.* Isolation of a Neutral P8 Cluster via [2+2] Cycloaddition of a Diphosphene Facilitated by Carbene Activation of White Phosphorus. Angew. Chem. Int. Ed. 2013, 52, 4462–4465. With VIP distinction and inside front cover. Link to article Featured on inside front cover
Mushinski, R. M.; Squires, B. M.; Sincerbox, K. A.; Hudnall, T. W.* Amino-Acrylamido Carbenes: Modulating Carbene Reactivity via Decoration with an α,β-unsaturated Carbonyl Moiety. Organometallics 2012, 31, 4862-7870. Link to article

